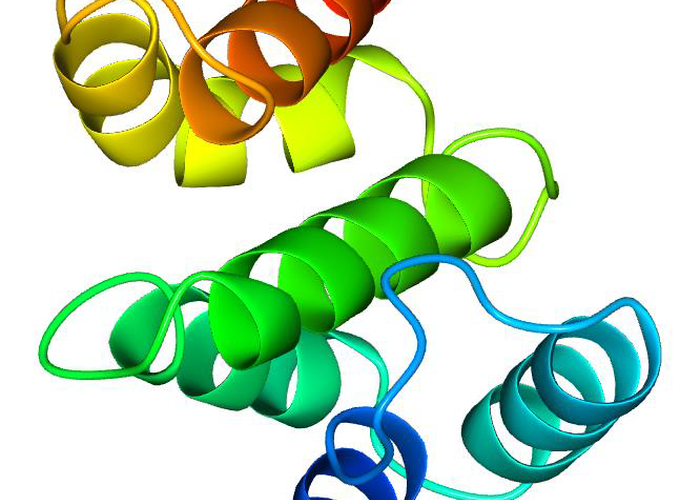
 VHS-Like Domain of Tepsin
VHS-Like Domain of Tepsin
 VHS-Like Domain of Tepsin
VHS-Like Domain of Tepsin
Adaptor protein 4 (AP4) is a critical intracellular trafficking protein complex in mammalian organisms that also plays a key role in the nervous system. A mutation in any of the four subunits of AP4 leads to a syndrome of debilitating diseases called hereditary spastic paraplegias. Tepsin is the only known accessory protein involved with AP4 in intracellular trafficking. Tepsin is composed of two ordered domains, one N-terminal and one central, and two C-terminal conserved motifs. These motifs are used to interact with the eta and beta-4 subunits of AP4 in an essential manner such that tepsin and AP4 exist synchronously and have evolved together. The biological function of tepsin is not presently known. As part of the Jackson Lab, I was on the team that determined the high-resolution x-ray crystal structure of the VHS-like domain of tepsin (tVHS) to a resolution of 1.86 Å. Circular dichroism spectroscopy (CD) was used to further test the structural features of tVHS. While there are significant similarities to other intracellular trafficking proteins that are involved in protein cargo binding and ubiquitin recognition, specific structural features on tVHS explain the biochemical experimental results that the Jackson Lab had previously conducted. While tVHS has structural similarities to other VHS domains it does not bind ubiquitin or acidic dileucine motifs.
Paper located here.